New Publication in PNAS
In collaboration with researchers at the NIH, Associate professor Katrine T Schjoldager and colleagues from the Copenhagen Center for Glycomics have identified a molecular mechanism that may contribute to the development and progression of chronic kidney disease (CKD).
Abstract
Chronic kidney disease (CKD) affects more than 20 million Americans and approximately 10% of the population worldwide. Genome-wide association studies (GWAS) of kidney functional decline have identified genes associated with CKD, but the precise mechanisms by which they influence kidney function remain largely unexplored. Here, we examine the role of one GWAS-identified gene by creating mice deficient in Galnt11, which encodes a member of the enzyme family that initiates protein O-glycosylation, an essential posttranslational modification known to influence protein function and stability. We find that Galnt11-deficient mice display low-molecular-weight proteinuria and have specific defects in proximal tubule-mediated resorption of the vitamin D binding protein, α1-microglobulin, and retinol binding protein. Moreover, we identify the endocytic receptor megalin (LRP2) as a direct target of Galnt11 in vivo. Megalin in Galnt11-deficient mice displays reduced ligand binding and undergoes age-related loss within the kidney. Differential mass spectrometry revealed specific sites of Galnt11-mediated glycosylation within mouse kidney megalin/LRP2 that are known to be involved in ligand binding, suggesting that O-glycosylation directly influences the ability to bind ligands. In support of this, recombinant megalin containing these sites displayed reduced albumin binding in cells deficient in Galnt11. Our results provide insight into the association between GALNT11 and CKD and identify a role of Galnt11 in proper kidney function.
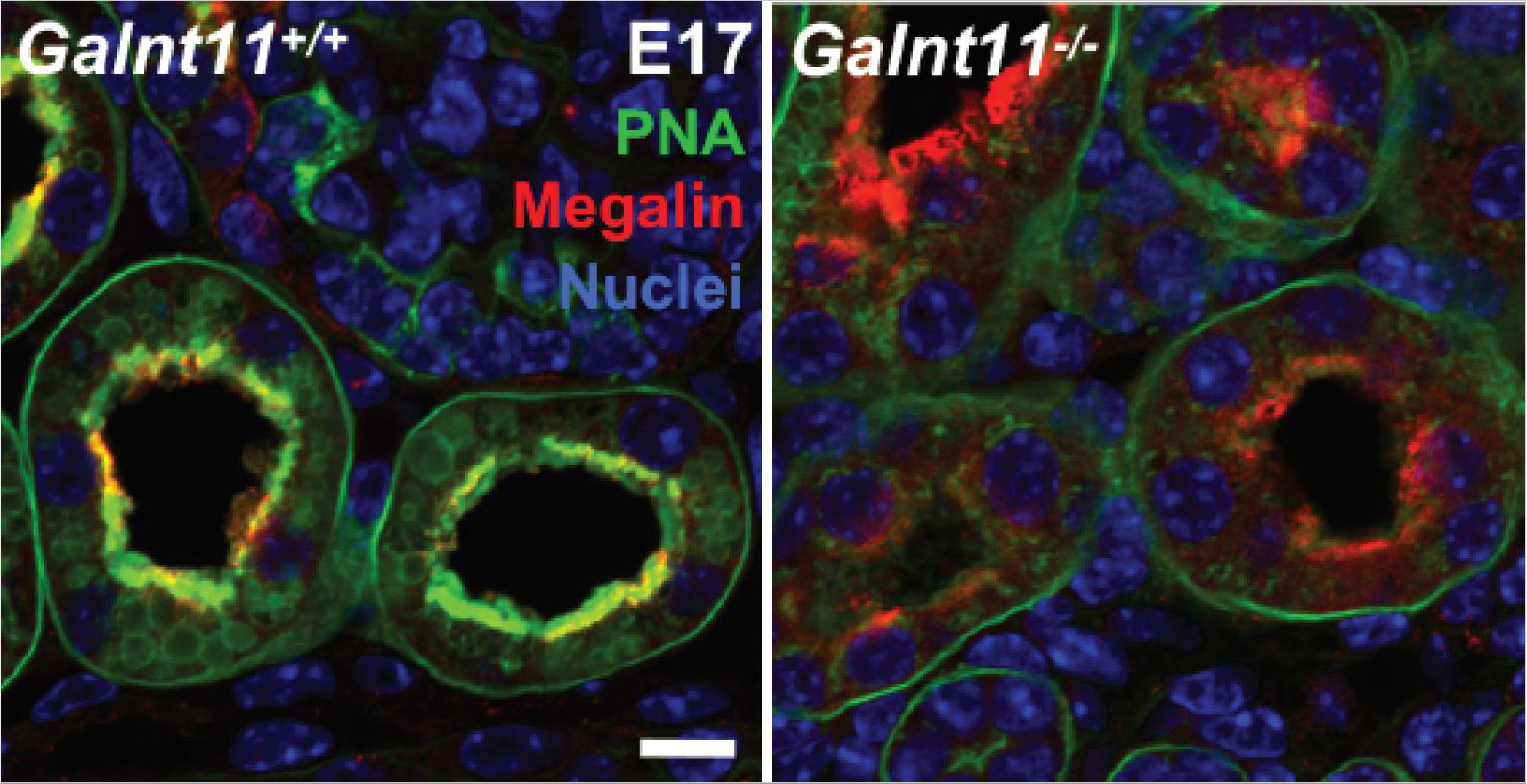
Read the full publication
E. Tian, Shengjun Wang, Liping Zhang, Ying Zhang, May C. Malicdan, Yang Mao, Christina Christoffersen, Lawrence A. Tabak, Katrine T. Schjoldager, and Kelly G. Ten Hagen. (2019) Galnt11 regulates kidney function by glycosylating the endocytosis receptor megalin to modulate ligand binding. PNAS. doi: 10.1073/pnas.1909573116
