Glycoengineering of NK Cells for B-Cell Lymphoma Cancer Therapy
CCG Director and Professor Henrik Clausen and Assistant Professor Yoshiki Narimatsu team up with collaborating researchers to glycoengineer NK cells with glycan ligands of CD22 and selectins to promote both targeted killing of B lymphoma cells and improved trafficking to sites where the cancer cells reside. Hong et al. (2020) found that these killer cells armed with CD22‐ligands exhibit significantly enhanced binding and killing of B‐lymphoma cells in vitro. In summary, cell‐surface chemoenzymatic glycan editing offers an easy‐to‐practice strategy to boost the efficacy of therapeutic cells for better cancer treatment. Despite glycan turnover and cell proliferation, a significant portion of CD22 ligands still remain on the cell surface after 72 hrs, which is in line with the persistence time of irradiated NK‐92MI cells in human patients. Therefore, this technique provides a nice complement or synergistic approach to the permanent, genetic engineering approach that has found great success in constructing NK‐92MI‐based CAR‐NK cells.
Publication Title:
Glycoengineering of NK Cells with Glycan Ligands of CD22 and Selectins for B-Cell Lymphoma Therapy
Publication Abstract:
CD22, a member of Siglec family of sialic acid binding proteins, has restricted expression on B cells. Antibody-based agents targeting CD22 or CD20 on B lymphoma and leukemia cells exhibit clinical efficacy for treating these malignancies, but also attack normal B cells leading to immune deficiency. Here, we report a chemoenzymatic glycocalyx editing strategy to introduce high-affinity and specific CD22 ligands onto NK-92MI and cytokine-induced natural killer cells to achieve tumor-specific CD22 targeting. These CD22-ligand modified cells exhibited significantly enhanced tumor cell binding and killing in vitro without harming healthy B cells. For effective lymphoma cell killing in vivo, we further functionalized CD22 ligand-modified NK-92MI cells with the E-selectin ligand sialyl Lewis X to promote trafficking to bone marrow. The dual-functionalized cells resulted in the efficient suppression of B lymphoma in a xenograft model. Our results suggest that nature killer cells modified with glycan ligands to CD22 and selectins promote both targeted killing of B lymphoma cells and improved trafficking to sites where the cancer cells reside, respectively.
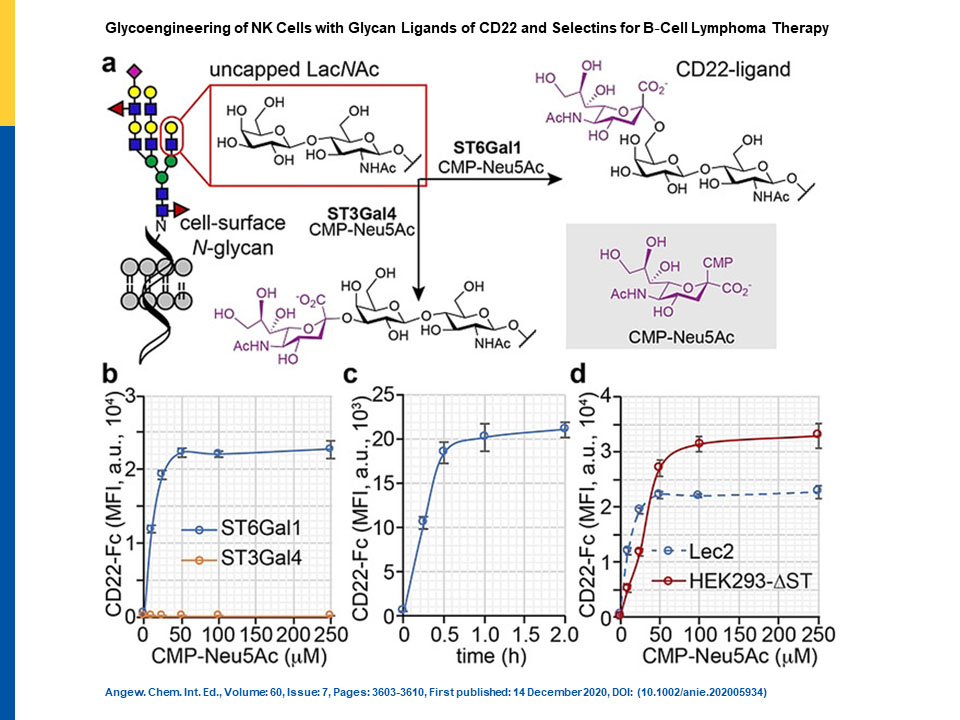
Figure 1
Chemo‐enzymatic generation of sialyl ligands on live cells to increase CD22 binding. a) ST6Gal1‐ and ST3Gal4‐assisted incorporation of Neu5Ac onto type 2 LacNAc in α2‐6‐ and α2‐3‐linkages, respectively. The resulting Neu5Acα2‐6Galβ1‐4GlcNAc is a natural ligand of CD22, whereas Neu5Acα2‐3Galβ1‐4GlcNAc is not. b–d) Flow cytometry quantification of CD22‐Fc binding of ST‐engineered live cells. The error bars represent the standard deviation of three biological replicates. b) Creation of CD22 ligands on Lec2 cells by STS‐mediated glycan editing was assessed with CD22‐Fc. c) Using CD22‐Fc to probe time‐dependent creation of CD22 ligands on Lec2 cells by ST6Gal1‐mediated glycan editing. d) Comparison of CD22‐Fc binding of Lec2 and HEK293‐ΔST mutant cells modified by ST6Gal1.
Citation:
Hong S, Yu C, Wang P, Shi Y, Cao W, Cheng B, Chapla DG, Ma Y, Li J, Rodrigues E, Narimatsu Y, Yates JR 3rd, Chen X, Clausen H, Moremen KW, Macauley MS, Paulson JC, Wu P. Glycoengineering of NK Cells with Glycan Ligands of CD22 and Selectins for B-Cell Lymphoma Therapy. Angew Chem Int Ed Engl. 2020 Dec 14. doi: 10.1002/anie.202005934. Epub ahead of print. PMID: 33314603.
