New PNAS Publication by Henrik Clausen et al.
Structure-guided engineering of the affinity and specificity of CARs against Tn-glycopeptides
Authors:
Preeti Sharma, Venkata V. V. R. Marada, Qi Cai, Monika Kizerwetter, Yanran He, Steven P. Wolf, Karin Schreiber, Henrik Clausen, Hans Schreiber, and David M. Kranz
Summary:
CAR T cells have shown significant promise in treating hematopoietic cancers but challenges remain, including antigen loss and identification of targets in solid tumors. Aberrant O-linked glycosylation in solid cancers is common, often resulting in expression of cell-surface neoantigens. We used directed evolution to engineer the binding site of an antibody so variants reacted more broadly with tumor-specific glycoprotein epitopes containing Tn (GalNAc-O-S/T). As CARs, these variants showed improved broader activity against mouse and human cancer cells with dysregulated O-glycosylation. Even cancer cells without MUC1, the most studied cancer-associated glycoantigen, were recognized by the engineered CARs. Cancer-specific recognition was retained due to Tn-antigen requirement, but broader activity against the glycoprotein backbone provides opportunities for potent use with minimal antigen-loss escape.
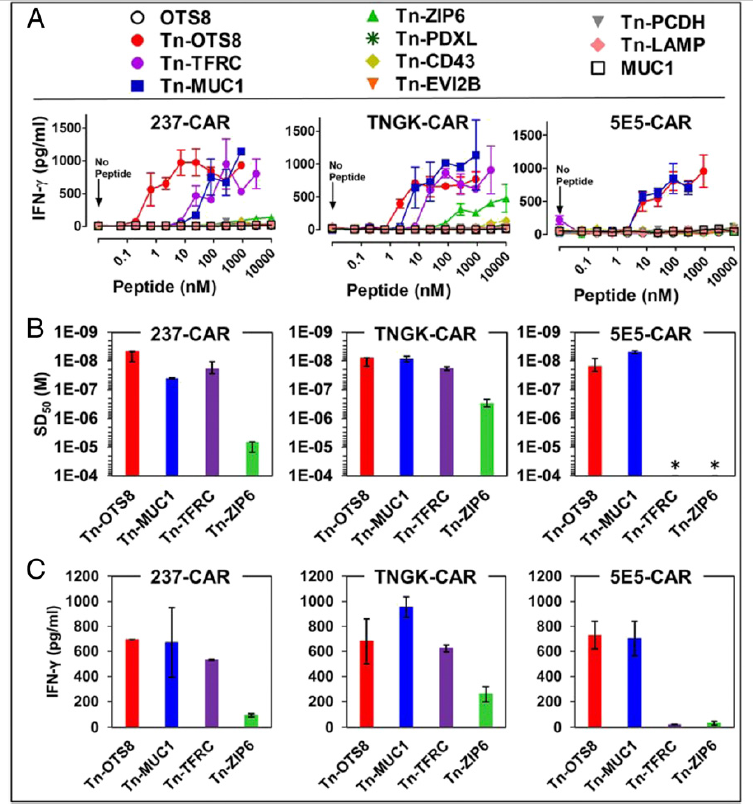
Fig. 6. Activity of 237-derived CAR T cells against additional human Tn-peptides. (A) To assess activation, mock, 237-CAR, TNGK-CAR, or 5E5-CAR transduced T cells were added to plates containing various concentrations of the indicated Tn-peptides and incubated at 37 °C, 5% CO2. After 24 h, IFN-γ released in the culture supernatants was measured by ELISA. Activation of transduced cells under each condition was measured in duplicate in two independent experiments.
Error bars indicate SEM. (B) Sensitization doses that yielded half-maximal stimulation (SD50) of IFN-γ release were calculated for the active Tn-peptides using nonlinear regression analysis (GraphPad Prism 5). 5E5-CAR was not activated with TFRC and ZIP6; hence the SD50 values were assigned values of >10−4 M (indicated by an *). SD50 values from two independent experiments are plotted as replicates. (C) Maximum IFN-γ release for the active peptides was determined at the 1-μM concentration. Mean ± SD is plotted for B and C.
Reference:
Sharma P, Marada VVVR, Cai Q, Kizerwetter M, He Y, Wolf SP, Schreiber K, Clausen H, Schreiber H, Kranz DM (2020). Structure-Guided Engineering of the Affinity and Specificity of CARs Against Tn-Glycopeptides. Proc Natl Acad Sci U S A. Jun 15:201920662. doi: 10.1073/pnas.1920662117
