Stability of bone-derived hormone osteocalcin could be improved in humans to enhance therapeutic effect
eLife Digest
Bones provide support and protection for organs in the body. However, over the last 15 years researchers have discovered that bones also release chemicals known as hormones, which can travel to other parts of the body and cause an effect. The cells responsible for making bone, known as osteoblasts, produce a hormone called osteocalcin which communicates with a number of different organs, including the pancreas and brain. When osteocalcin reaches the pancreas, it promotes the release of another hormone called insulin which helps regulate the levels of sugar in the blood. Osteocalcin also travels to other organs such as muscle, where it helps to degrade fats and sugars that can be converted into energy. It also has beneficial effects on the brain, and has been shown to aid memory and reduce depression. Osteocalcin has largely been studied in mice where levels are five to ten times higher than in humans. But it is unclear why this difference exists or how it alters the role of osteocalcin in humans. To answer this question, Al Rifai et al. used a range of experimental techniques to compare the structure and activity of osteocalcin in mice and humans. The experiments showed that mouse osteocalcin has a group of sugars attached to its protein structure, which prevent the hormone from being degraded by an enzyme in the blood. Human osteocalcin has a slightly different protein sequence and is therefore unable to bind to this sugar group. As a result, the osteocalcin molecules in humans are less stable and cannot last as long in the blood. Al Rifai et al. showed that when human osteocalcin was modified so the sugar group could attach, the hormone was able to stick around for much longer and reach higher levels when added to blood in the laboratory. These findings show how osteocalcin differs between human and mice. Understanding this difference is important as the effects of osteocalcin mean this hormone can be used to treat diabetes and brain disorders. Furthermore, the results reveal how the stability of osteocalcin could be improved in humans, which could potentially enhance its therapeutic effect.
Publication Title:
The half-life of the bone-derived hormone osteocalcin is regulated through O-glycosylation in mice, but not in humans
Publication Abstract:
Osteocalcin (OCN) is an osteoblast-derived hormone with pleiotropic physiological functions. Like many peptide hormones, OCN is subjected to post-translational modifications (PTMs) which control its activity. Here, we uncover O-glycosylation as a novel PTM present on mouse OCN and occurring on a single serine (S8) independently of its carboxylation and endoproteolysis, two other PTMs regulating this hormone. We also show that O-glycosylation increases OCN half-life in plasma ex vivo and in the circulation in vivo. Remarkably, in human OCN (hOCN), the residue corresponding to S8 is a tyrosine (Y12), which is not O-glycosylated. Yet, the Y12S mutation is sufficient to O-glycosylate hOCN and to increase its half-life in plasma compared to wildtype hOCN. These findings reveal an important species difference in OCN regulation, which may explain why serum concentrations of OCN are higher in mouse than in human.
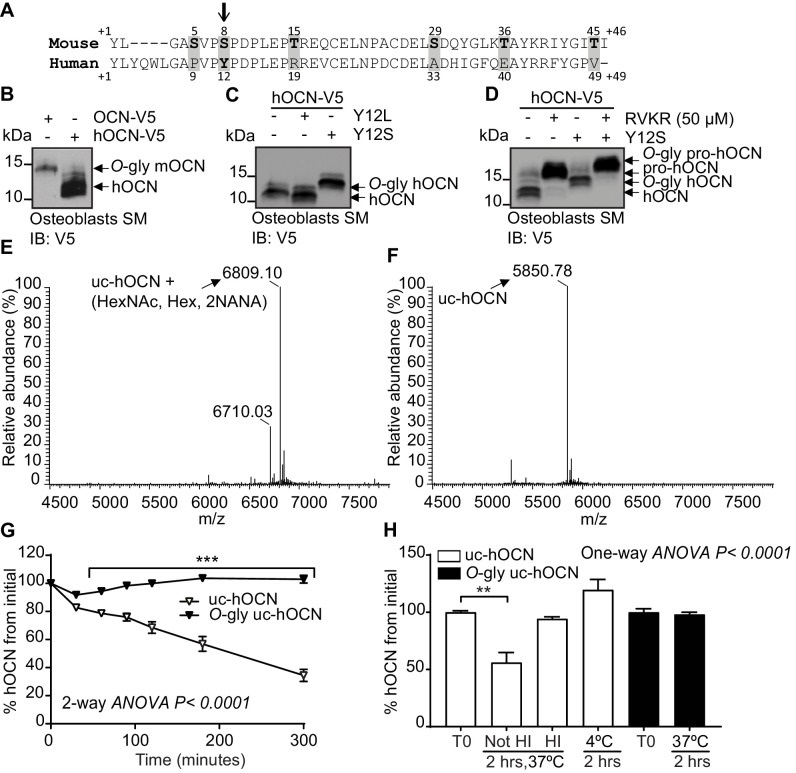
Figure 5
Human OCN O-glycosylation increases its half-life ex vivo. (A) Amino acid alignment of mouse and human OCN. The six serine and threonine residues present in the mouse protein and their corresponding amino acids in human OCN are highlighted in gray. The site of O-glycosylation in mouse OCN (S8) is indicated by an arrow. (B) Western Blot analysis on the secretion media (SM) of primary osteoblasts transfected with human OCN-V5 (hOCN) and mouse OCN-V5 (OCN). (C) Western blot analysis on the SM of primary osteoblasts transfected with hOCN-V5 containing or not the indicated mutations. (D) Western Blot analysis on the SM of primary osteoblasts transfected with hOCN-V5 containing or not the Y12S mutations and treated or not with 50 μM Dec-RVKR-CMK (RVKR). (E) Annotated and deconvoluted MS spectrum of purified O-glycosylated uncarboxylated human OCN (O-gly uc-hOCN). (F) Annotated and deconvoluted MS spectrum of and purified non-glycosylated uncarboxylated human OCN (uc-hOCN). (G–H) Ex vivo half-life of O-gly uc-hOCN and uc-hOCN in mouse plasma. (G) 60 ng/ml of O-gly uc-hOCN and uc-hOCN were incubated at 37°C in plasma of OCN deficient mice (Bglap-/-) (n = 4) for 0 to 5 hr and hOCN levels were measured at the indicated time. (H) O-gly uc-hOCN and uc-hOCN were incubated in Bglap-/- plasma for 2 hr at 37°C in different conditions or at 4°C (n = 4 independent plasma per condition). T0: start point, see Materials and methods; HI: heat-inactivated plasma; HexNAc: N-acetylhexosamine; Hex: Hexose; NANA: N-acetylneuraminic acid. Uc-hOCN levels were measured at the indicated time points using an uc-hOCN ELISA assay. Results are given as mean ± SEM. **p<0.01; ***p<0.001 using two-way ANOVA for repeated measurements with Bonferroni multiple comparisons test.
Citation:
Al Rifai O, Julien C, Lacombe J, Faubert D, Lira-Navarrete E, Narimatsu Y, Clausen H, Ferron M. The half-life of the bone-derived hormone osteocalcin is regulated through O-glycosylation in mice, but not in humans. Elife. 2020 Dec 7;9:e61174. doi: 10.7554/eLife.61174. PMID: 33284103; PMCID: PMC7822592.
