New publication in Blood Advances
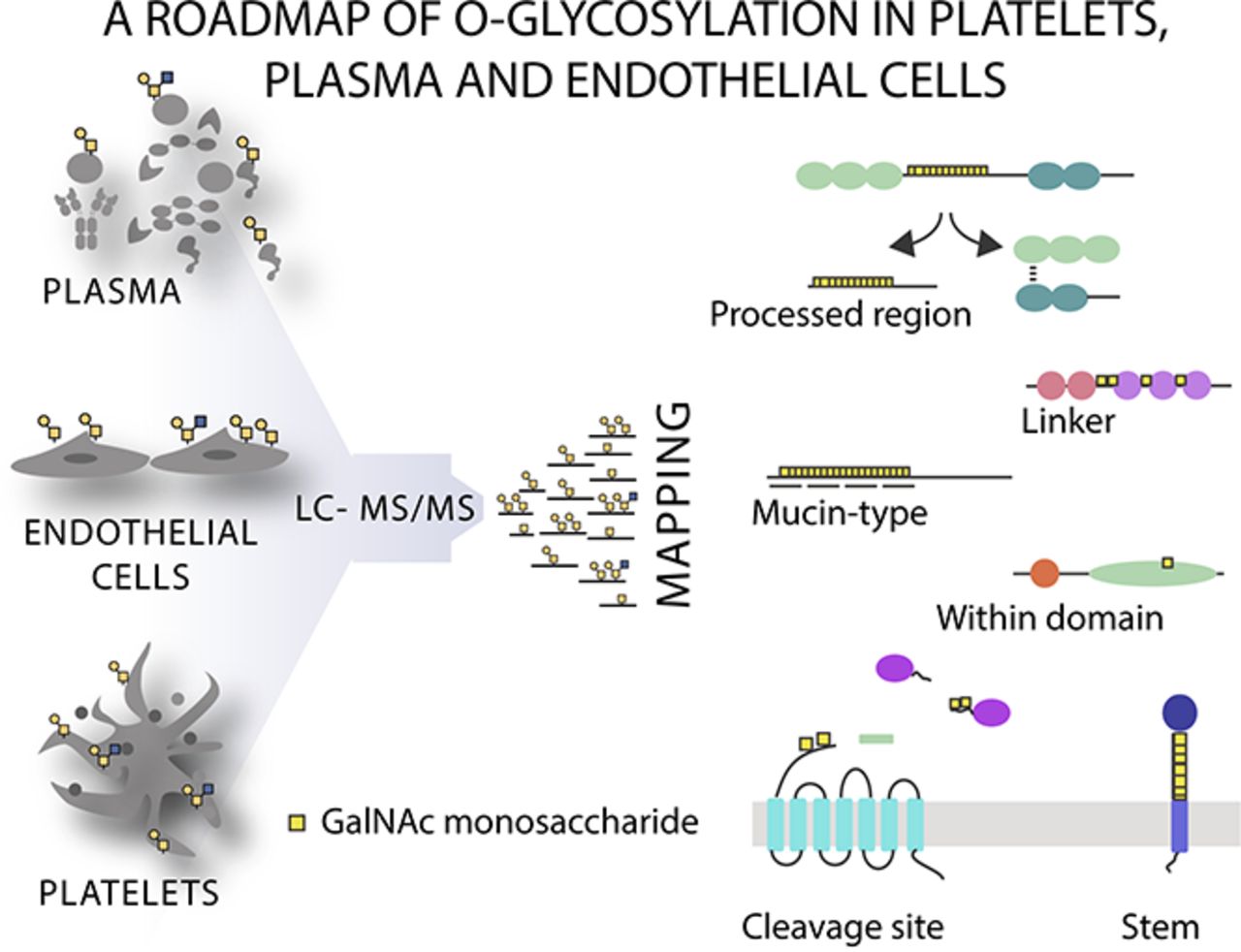
The hemostatic system comprises platelet aggregation, coagulation, and fibrinolysis, and is critical to the maintenance of vascular integrity. Here we performed the first systematic analysis of native-O-glycosylation using lectin affinity chromatography coupled to liquid chromatography mass spectrometry (LC-MS)/MS to determine the precise location of O-glycans in human plasma, platelets, and endothelial cells, which coordinately regulate hemostasis. We identified the hitherto largest O-glycoproteome from native tissue with a total of 649 glycoproteins and 1123 nonambiguous O-glycosites, demonstrating that O-glycosylation is a ubiquitous modification of extracellular proteins. Using an unbiased screen to identify associations between O-glycosites and protein annotations we found that O-glycans were over-represented close (± 15 amino acids) to tandem repeat regions, protease cleavage sites, within propeptides, and located on a select group of protein domains. The importance of O-glycosites in proximity to proteolytic cleavage sites was further supported by in vitro peptide assays demonstrating that proteolysis of key hemostatic proteins can be inhibited by the presence of O-glycans. Collectively, these data illustrate the global properties of native O-glycosylation and provide the requisite roadmap for future biomarker and structure-function studies.
King SL, Joshi HJ, Schjoldager KT, Halim A, Madsen TD, Dziegiel MH, Woetmann A, Vakhrushev SY and Wandall HH (2017): Characterizing the O-glycosylation landscape of human plasma, platelets, and endothelial cells. Blood Advances 1:429-442.
