Coronavirus Uses Heparan Sulfate to Get Inside Cells
Authors: Thomas Mandel Clausen, Daniel R. Sandoval, Charlotte B. Spliid, Jessica Pihl, Hailee R. Perrett, Chelsea D. Painter, Anoop Narayanan, Sydney A.Majowicz, Elizabeth M.Kwong, Rachael N. McVicar, Bryan E.Thacker, Charles A. Glass, Zhang Yang, Jonathan L. Torres, Gregory J. Golden, Phillip L. Bartels, Ryan N. Porell, Aaron F. Garretson,… Jeffrey D. Esko
 Highlights
Highlights
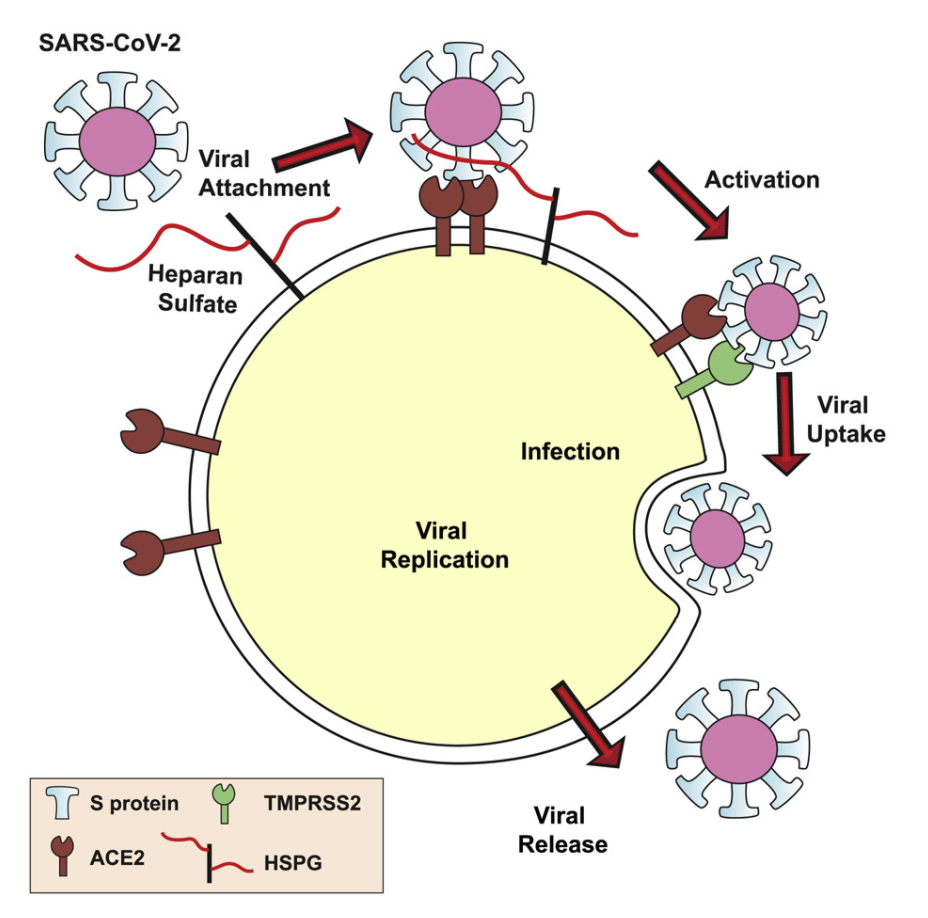
- SARS-CoV-2 spike protein interacts with heparan sulfate and ACE2 through the RBD
- Heparan sulfate promotes Spike-ACE2 interaction
- SARS-CoV-2 infection is co-dependent on heparan sulfate and ACE2
- Heparin and non-anticoagulant derivatives block SARS-CoV-2 binding and infection
Summary
We show that SARS-CoV-2 spike protein interacts with both cellular heparan sulfate and angiotensin-converting enzyme 2 (ACE2) through its receptor-binding domain (RBD). Docking studies suggest a heparin/heparan sulfate-binding site adjacent to the ACE2-binding site. Both ACE2 and heparin can bind independently to spike protein in vitro, and a ternary complex can be generated using heparin as a scaffold. Electron micrographs of spike protein suggests that heparin enhances the open conformation of the RBD that binds ACE2. On cells, spike protein binding depends on both heparan sulfate and ACE2. Unfractionated heparin, non-anticoagulant heparin, heparin lyases, and lung heparan sulfate potently block spike protein binding and/or infection by pseudotyped virus and authentic SARS-CoV-2 virus. We suggest a model in which viral attachment and infection involves heparan sulfate-dependent enhancement of binding to ACE2. Manipulation of heparan sulfate or inhibition of viral adhesion by exogenous heparin presents new therapeutic opportunities.
Click here to read more online
Click here to view news story from the Faculty of Health and Medical Sciences. (no longer available)
