New CCG Publication Provides Quantitative Characterization of O-GalNAc Glycosylation
O-GalNAc type glycosylation is one of the most abundant and diverse protein modifications. Postdoc Tomislav Caval together with postdoc Noortje de Haan and PhD student Andriana Konstantinidi, under the guidance of associate professor Sergey Vakhrushev, highlight that quantitative characterization of O-GalNAc glycosylation can be addressed at the glycan, glycopeptide and glycoprotein level.
Publication Abstract:
O-GalNAc type glycosylation is an abundant and complex protein modification. Recent developments in mass spectrometry resulted in significant success in quantitative analysis of O-GalNAc glycosylation. The analysis of released O-GalNAc type glycans expanded our horizons of understanding the glycome of various biological models. The site-specific analysis of glycosylation micro-heterogeneity of purified proteins opened perspectives for the improved design of glycoprotein therapeutics. Advanced gene editing and chemical technologies applied to O-glycoproteomics enabled to identify O-GalNAc glycosylation at unprecedented depth. Progress in the analysis of intact glycoproteins under native and reduced conditions enabled the monitoring of glycosylation proteoform variants. Despite of the astonishing results in quantitative O-GalNAc glycoproteomics, site-specific mapping of the full O-GalNAc structural repertoire in complex samples is yet a long way off. Here, we summarize the most common quantitative strategies in O-GalNAc glycoproteomics, review recent progress and discuss benefits and limitations of the various approaches in the field.
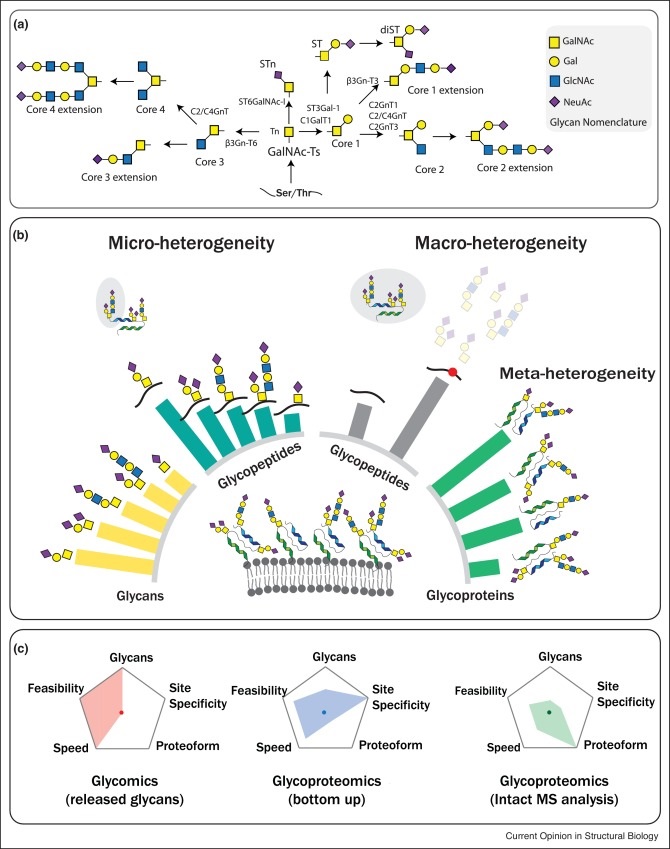
Figure 1. Overview of quantitative strategies in O-GalNAc glycoproteomics.
(a) The most common steps of O-GalNAc glycosylation pathway with the enzymes involved in extension and branching of O-GalNAc structures. (b) Schematic presentation of quantitative analysis at three different levels (glycans, glycopeptides and glycoproteins). (c) Comparison of analytical potential of glycomics, bottom up glycoproteomics and intact MS glycoproteomics approaches.
Citation:
Čaval T, de Haan N, Konstantinidi A, Vakhrushev S. “Quantitative Characterization of O-GalNAc Glycosylation.” Current opinion in structural biology 68 (2021): 135–141. Web.
