New JBC Publication: Genetic Glycoengineering in Mammalian Cells
Publication Title: Genetic glycoengineering in mammalian cells
Authors: Yoshiki Narimatsu, Christian Bull, Yen-Hsi Chen, Hans H. Wandall, Zhang Yang, and Henrik Clausen
Publication Abstract:
Advances in nuclease-based gene-editing technologies have enabled precise, stable, and systematic genetic engineering of glycosylation capacities in mammalian cells, opening up a plethora of opportunities for studying the glycome and exploiting glycans in biomedicine. Glycoengineering using chemical, enzymatic and genetic approaches has a long history, and precise gene editing provides a nearly unlimited playground for stable engineering of glycosylation in mammalian cells to explore and dissect the glycome and its many biological functions. Genetic engineering of glycosylation in cells also brings studies of the glycome to the single cell level, and opens up wider use and integration of data in traditional omics workflows in cell biology. The last few years have seen new applications of glycoengineering in mammalian cells with perspectives for wider use in basic and applied glycosciences, and these have already led to discoveries of functions of glycans and improved designs of glycoprotein therapeutics. Here, we review the current state-of-the-art of genetic glycoengineering in mammalian cells and highlight emerging opportunities.
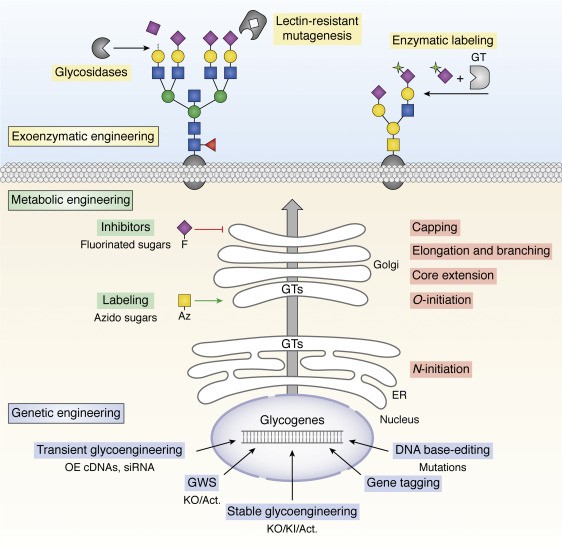
Figure 1. Overview of glycoengineering strategies. Basic principles for approaches available to modulate the cellular glycosylation processes and the glycome are illustrated. Extracellularly, glycans may be modulated by more or less selective endo-/exoglycosidases (sialidases, galactosidases, PNGase, etc.) (117), and chemoenzymatic labeling methods utilizing e.g. glycosyltransferases (GTs) may be applied to install natural or unnatural substrates on cell surface glycans (6,337). Use of cytotoxic lectins often in combination with mutagenesis may enable selection of mutant cells with loss/gain of distinct glycosylation features (10,338). A growing number of unnatural sugar mimetics can be applied for metabolic engineering (6,23), including glycosylation inhibitors (i.e. fluorinated sugar analogues) (235,339) or functionalized sugars (i.e. azido, Az, sugars) that enable conjugation chemistries for use in glycan imaging or reprogramming of their interactions (20,160). Genetic engineering of glycosylation may be performed by overexpression (OE) of GTs using cDNA plasmid transfection and/or siRNA for silencing of endogenous GTs (6). More extensive and stable glycoengineering take advantage of precise gene engineering for combinatorial KO/KI/Act (activation) of GTs, and this strategy is the main focus of this review. Genome-wide KO/Act screens (GWS) may be used for discovery and dissection of GTs and other genes affecting glycosylation (Table 1), and endogenous GTs may be mutated, e.g. to mimic disease mutations or enable use of unique substrates, or tagged, e.g. by insertion of antibody tags or fluorescent proteins (169).
Citation:
Narimatsu, Yoshiki et al. “Genetic Glycoengineering in Mammalian Cells.” The Journal of biological chemistry (2021): 100448–100448. Web.
